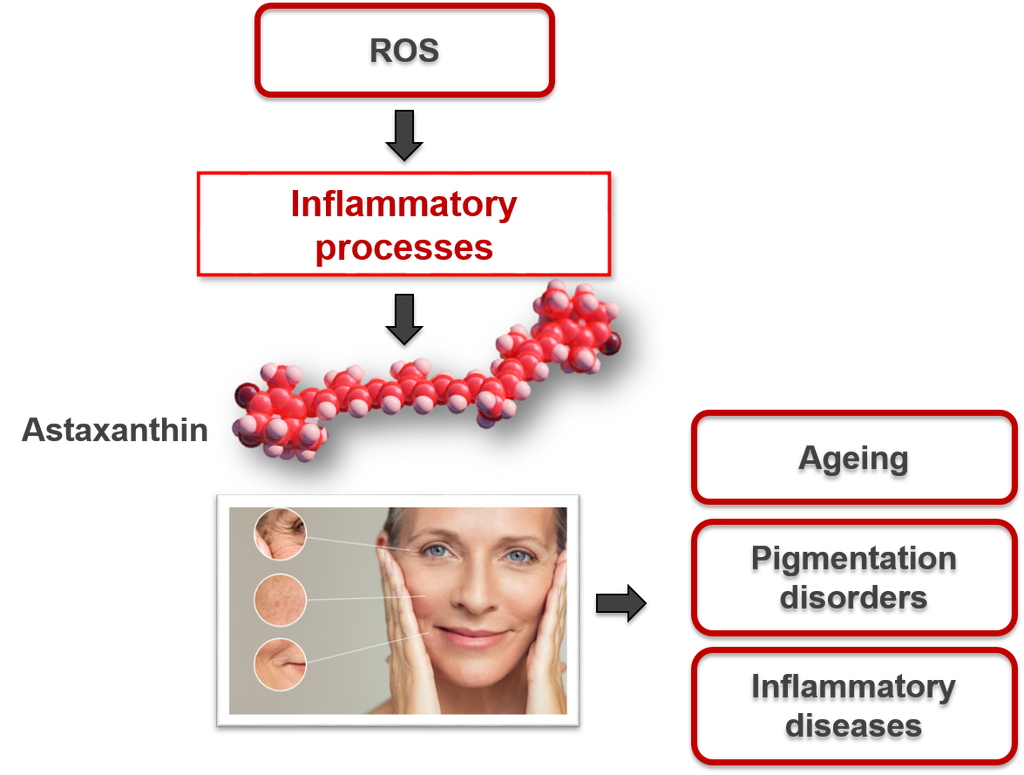
Astaxanthin – biological cell protection against light-induced skin damage
An efficient antioxidant that provides biological cell protection against light-induced skin damage
Abstract:
Exposure to solar radiation is a major cause of physiological skin damage by promoting the formation of free radicals and triggering oxidative stress. Harmful UV irradiation leads to inflammation, accelerated skin ageing and malignant skin disease. Therefore, we need to protect our outermost barrier from harmful solar radiation to reduce light-induced skin changes.
Astaxanthin, a naturally occurring reddish pigment derived from the microalga Haematococcus pluvialis, is considered the most powerful natural antioxidant with no pro-oxidant or phototoxic character, highly effective in counteracting reactive oxygen species (ROS).
Numerous studies have shown astaxanthin’s positive effect on human skin. Due to its high antioxidant and antiinflammatory properties, it supports the skin’s oxidative balance, inhibits inflammatory processes and provides natural protection of the human skin against a negative impact of UV rays. Furthermore, astaxanthin supports the skin’s biological cell protection against light-induced damage without providing a sun protection factor (SPF).
Introduction
Human skin, our body’s outermost protective barrier, is constantly exposed to an environment that promotes oxidation. wSunlight with its invasive UV and visual rays is particularly stressful for the skin. In the short term, light-induced oxidative stress leads to skin damage in the form of redness (erythema) and infl ammation. In the long term, accelerated ageing processes and serious consequences for the health of our skin, including skin cancer, are the result. Whenever our skin is exposed to intense photoradiation, changes occur in certain molecules within the body leading to the formation of various highly reactive oxygen species (ROS) and free radicals which cause oxidative stress (Figure 1) (1).
When ROS attack cell membranes, they activate enzymes that lead to cell death, changes in skin appearance and the onset of active skin ageing processes. They can also interfere with DNA and trigger a series of cellular processes that may induce infl ammation. This leads to a chain reaction causing various cell changes, including the destruction of collagen and other molecules that are vital for skin elasticity, ultimately leading to typical agerelated skin conditions (2).
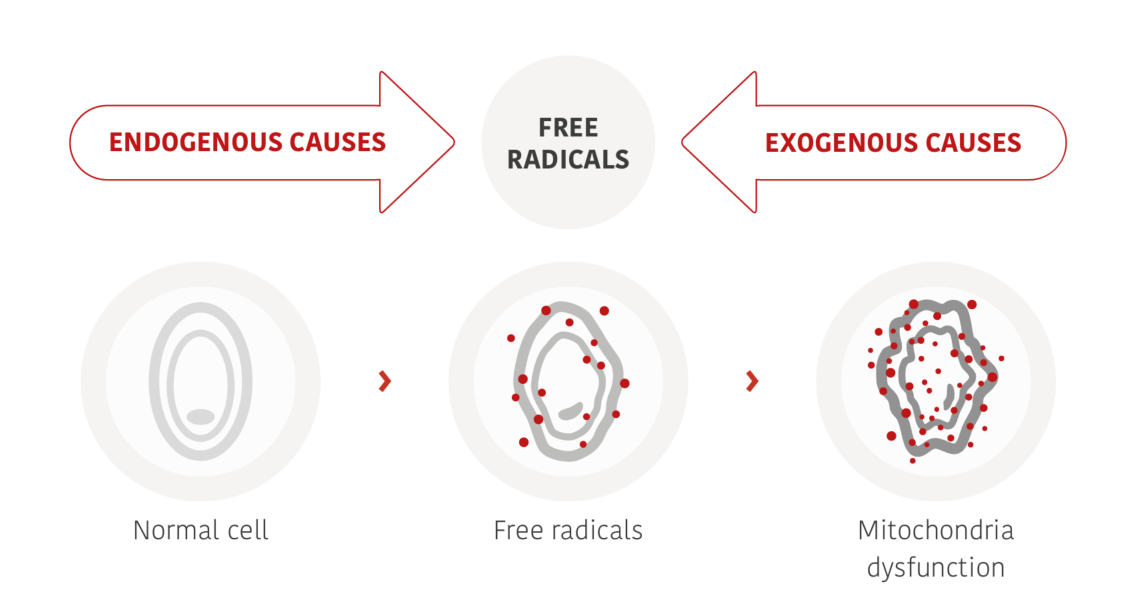
Figure 1: UV-induced ROS attack cell membranes and cause various changes in cellular structures and inflammation.
Light-induced skin damage
Depending on wavelength and energy level, different types of UV radiation can be distinguished, whereby UVA and UVB rays are mainly responsible for biological changes in the dermal tissue. Long-term and combined irradiation of human skin with UVB and UVA lead to a reduction and slowing down of the skin renewal potential as well as structural damage to the dermal matrix (Figure 2).
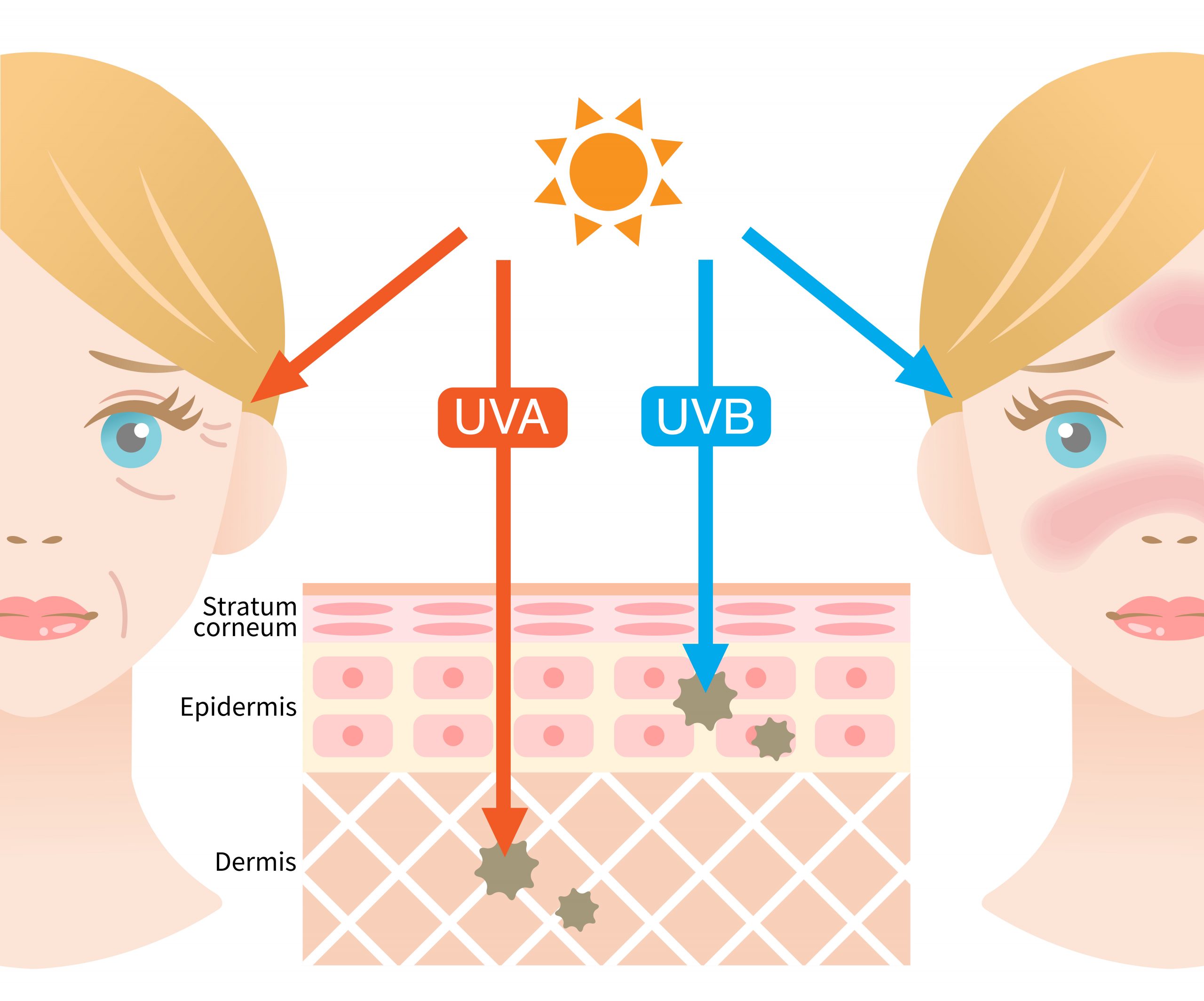
Figure 2: Mechanism of skin damage induced by different types of UV radiation.
UVA rays have a longer wavelength and cause long-term skin damage at all levels. By penetrating deep into the skin to the dermis, they trigger a breakdown of collagen and elastin, produced by fibroblasts and responsible for the skin’s fi rmness, elasticity and youthful tautness. UVB light causes the immediately visible tanning or reddening (sunburn) of the skin. UVB rays have a shorter wavelength and a high energy level. They mainly affect the outer layer of the skin – the epidermis – and harm epidermal keratinocytes and cellular macromolecules (e.g. lipids or proteins). This promotes the formation of ROS, promotes infl ammatory processes, damages DNA structures and impairs the skin’s ability to protect itself from exogenous and endogenous stressors. DNA damage associated with UV ray exposure are largely the result of errors in DNA repair, which can lead to mutations and ultimately increase skin cancer risk (3).
Therefore, it is important to protect our outermost barrier from harmful solar radiation for which broad-spectrum sun cream with a suitable sun protection factor (SPF) and good skin care are essential. However, sun creams cannot completely reduce free radical formation in the skin. Besides the use of sun creams, topical supplementation of antioxidants can provide additional protection at cellular levels against damaging reactive molecules (4).
Antioxidants against light-induced skin damage
Antioxidants are substances that protect the skin against oxidative stress by neutralising ROS. The human body naturally produces or absorbs antioxidants to prevent radical damage and maintain oxidative balance in the cells. Prolonged exposure to sunlight can overwhelm this innate defence system through overproduction of free radicals, leading to a state of oxidative imbalance. The incorporation of antioxidants into topical skin care products can provide additional protection and supplement the body’s innate defence to neutralise ROS (1).
When selecting antioxidants for skin care products, it should be considered that there are numerous antioxidants that may differ in their mechanism of action and in their antioxidative capacity. The strength of various antioxidants in neutralising singlet oxygen was determined by Nishida et al. (5). The antioxidants tested in this study were natural astaxanthin, coenzyme Q10, vitamins C and E as well many other known antioxidants. The results showed that astaxanthin is 6,000 times stronger than vitamin C, 790 times more powerful than CoQ10 and 110 times stronger than vitamin E in neutralising singlet oxygen. This is why astaxanthin is considered to be the most powerful antioxidant found in nature (5).
Natural Astaxanthin
Astaxanthin – the red diamond amongst antioxidants – is a naturally occurring reddish pigment derived from the microalga Haematococcus pluvialis (Figure 3), and belongs to the carotenoid group. Through its 13 conjugated double bonds, natural astaxanthin is highly effective at counteracting reactive oxygen species, plus, it has no prooxidative properties (1).
In several human studies, astaxanthin has shown to improve overall skin health and counteract skin ageing by reducing oxidative stress and providing comprehensive protection against ROS-related damage. Positive effects have been shown in particular with regard to elasticity, moisture, age spots and dermal texture. Astaxanthin improves skin elasticity by strengthening the collagen layer and revitalises photoaged tissue by eliminating free radicals in all skin layers (6, 7). In vitro and in vivo, an SPF effect has not been demonstrated for astaxanthin in topical formulations (8). Furthermore, astaxanthin has been shown to be nonphototoxic and therefore suitable to be used in sunscreens and sun care products (9).
Based on research findings as described above, we investigated the protective properties of astaxanthin in an in vitro and clinical model against oxidative stress and UV-induced erythema.
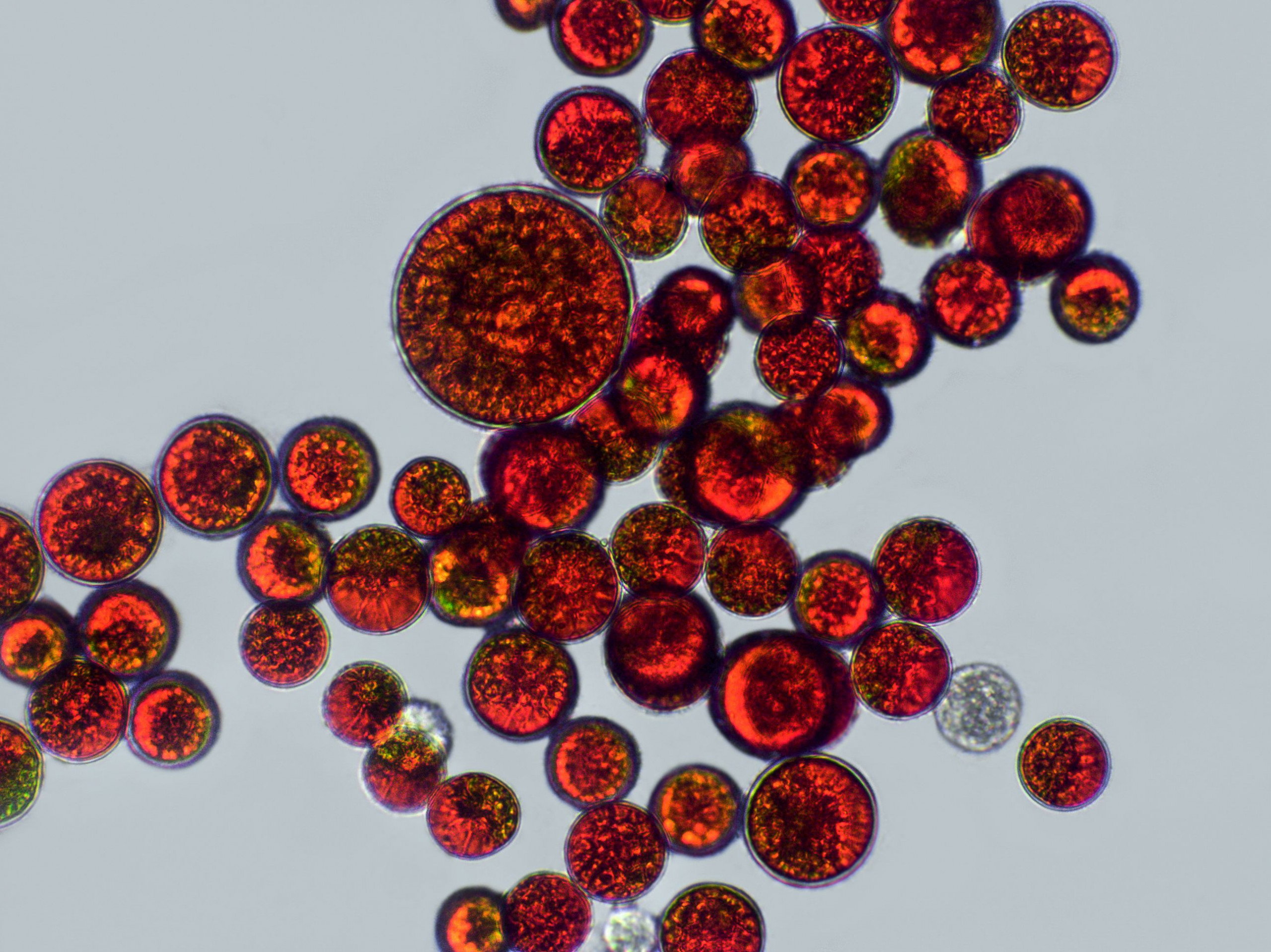
Figure 3. Astaxanthin-rich cells of the microalga Haematococcus pluvialis.
Methods
The first setting was a half-side and placebo-controlled pilot study with 21 healthy volunteers over 18 years (average age 22.61) with Fitzpatrick skin type 2 or 3. Before the study was launched, a UVB erythema test was carried out to determine the minimum erythema dose (MED) of each individual (the minimum dose causing sunburn). For the study, volar aspects of both forearms were irradiated with a light-testing system (Skintrek PT3 exposure device). Thirty minutes before UV irradiation, one forearm was treated with 1ml of an astaxanthin vehicle emulsion using 0.2% ASTACOS® OL50, the other with a placebo. ASTACOS® OL50 is a COSMOS-certified and Natrue approved vegan active ingredient containing 5% astaxanthin, specially developed for use in topical cosmetic formulations. In total, six treatment areas on each forearm were irradiated with gradually increasing intensities of UV radiation (steps of plus 0.05 J/cm²), starting with the MED value that induced a clearly visible reaction on the skin. Twenty-four hours after equal exposure to UV irradiation, the reaction on both forearms was photographed and evaluated visually using a 4-step scale (zero, +, ++, +++ redness) to classify the erythema level. In addition, erythema values were colorimetrically measured with the DSM II ColorMeter in all treated areas (10).
For the second study setting in vitro, primary human keratinocyte 3D skin models (human Phenion® 3D-full thickness skin model) were treated with an astaxanthin formulation with 0.05% ASTACOS® OL50 prior to distinct UVB irradiation at 200 mJ/cm² from a distance of approximately 20cm to avoid thermal effects. The test solution was applied topically to the 3D skin models one hour before irradiation. As a check, two reference tissues and two untreated irradiated 3D skin models were used. Twenty-four hours after irradiation, the skin models were processed for histological (tissue) assessment. Three 5-mm punch biopsies were taken from the centre of each 3D skin model, embedded in Tissue-Tek® O.C.TTM and then frozen in liquid nitrogen. The cryopreserved biopsies were cut into 6μm sections, fixed in acetone/ethanol and rehydrated before 30 to 50 sections of each model were stained with haematoxylin/eosin (H&E). The samples were visually analysed by a dermatologist for characteristic sunburn-related tissue damage (e.g. sunburned cells, hydropic cells, basal keratinocyte morphology/vacuolised keratinocytes) (11).
Results & Discussion
The visual evaluation of the photos taken 24 hours after UV irradiation (Figure 4) showed no erythema reaction (zero redness) in 6 of the 21 forearms treated with a placebo emulsion. On 6 of the placebo treated forearms a + erythema reaction, on other 6 forearms, a ++ erythema reaction, and in the remaining 3, a +++ erythema reaction was determined. This means that in 15 out of 21 forearms treated with a placebo, a visible reddening was detected. On the left forearms treated with astaxanthin emulsion, no erythematous reaction (zero redness) was observed in any of the subjects (Table 1).
These results correlate with the values measured with the DSM II ColorMeter. An average erythema value of 8.27 was measured on the side with astaxanthin (left forearms) and a value of 11.04 on the side with placebo (right forearms). Generally, a basic value of redness corresponding to the natural skin colour is also measured in non-UV-exposed skin, which is why the difference between the erythema values between right and left forearm is the value to be considered. In all 21 subjects, the degree of redness measured on the forearm treated with astaxanthin was lower than on the side with placebo, even in those subjects where no redness could be detected. The redness score on the astaxanthin-treated forearms was on average by 25% (2.76 points) lower than on the side with placebo.
In this setting, astaxanthin suppressed a visual erythema formation in more than 70% of cases. Topical application of astaxanthin showed biological cell protection and reduced the inflammatory effects of UVB radiation, which was expressed in reduced redness values of the skin. UV exposure causes inflammation, accelerated skin ageing and non-melanoma skin cancer. Topical astaxanthin reducing the inflammatory effects of UVB radiation could prevent later stages of photoageing and malignant skin disease like skin cancer (10).
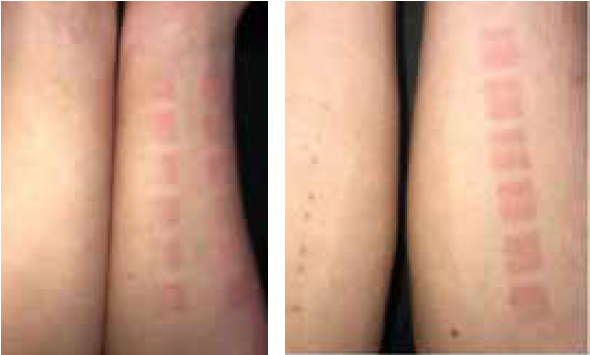
Figure 4: Photo documentation of erythema values in the exposed test areas on right and left forearms 24 hours after identical exposure to UV light on either side (right fore-arm: placebo, left forearm: topical astaxanthin): left forearm showing no erythema, right forearm showing erythema in the exposed test areas
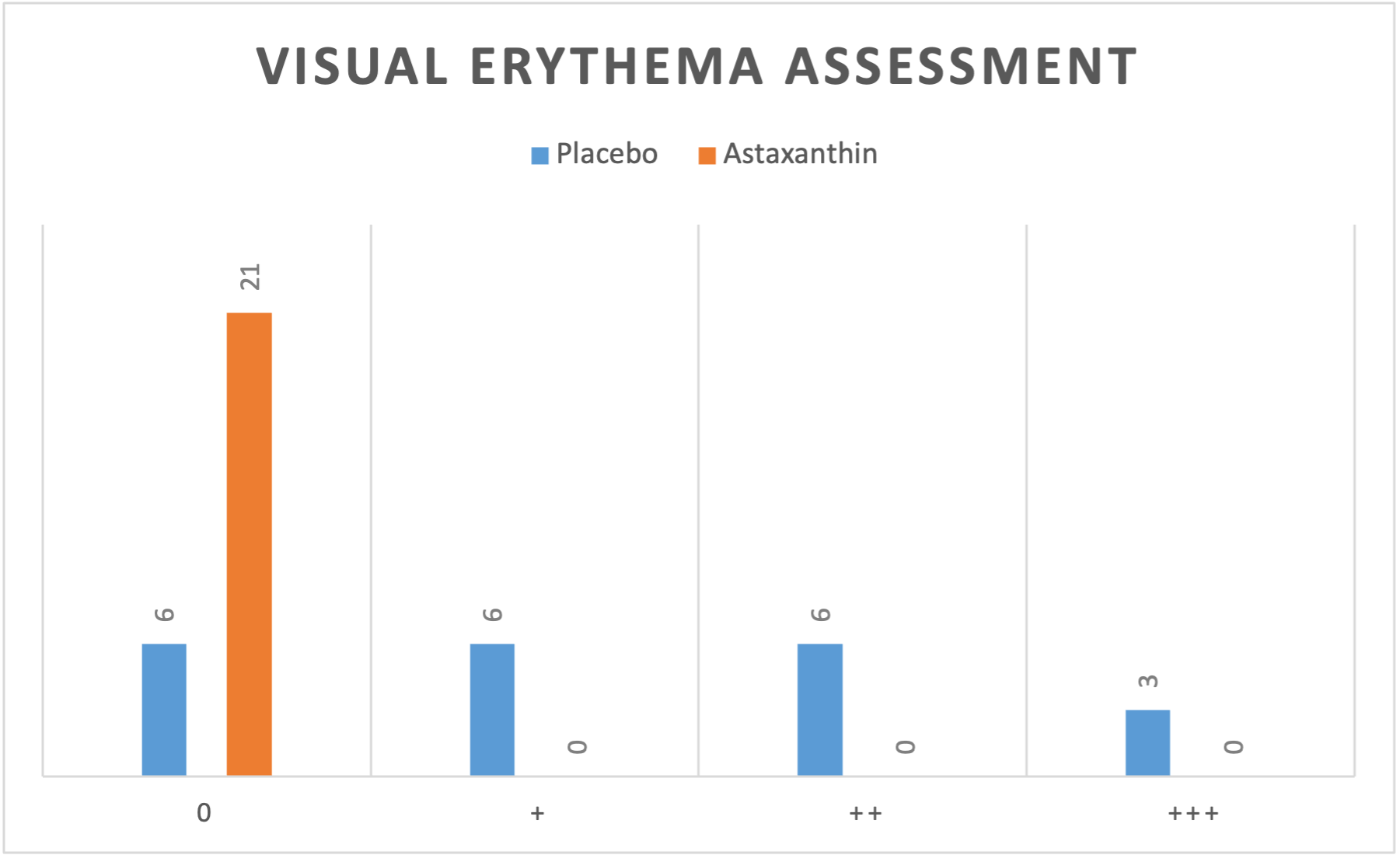
Table 1: Results of the visual assessment of erythema values in the exposed test areas 24 hours after identical exposure to UV light on either side (right forearm: placebo, left forearm: topical astaxanthin).
Astaxanthin also showed cell protective properties in keratinocytes against damage caused by light in vitro when exposed to intense UVB radiation. The untreated, irradiated models (Figure 5 area (B)) indicated clear signs of excessive UV irradiation and sunburned cells (Figure 5 area (B) yellow arrows) characterised by narrowed nuclei compared to the untreated, non-irradiated reference tissue (Figure 5 area (A)). In addition, hydropic cells (Figure 5 area (B) red arrows) with less intense staining can be identified which are the cause of cytosolic fluid accumulation. Vacuolated keratinocytes were found in the epidermis, reflecting typical tissue destruction after strong UV exposure as well as incipient parakeratosis. Compared to the untreated irradiated skin models, the tissue treated with the astaxanthin-containing test solution showed a much milder UVB phenotype without parakeratosis (Figure 5 area (C)). The tissue was less vacuolated and contained fewer apoptotic cells and vacuolated keratinocytes. Significantly less sunburned cells (Figure 5 area C, yellow arrows) and apoptotic cells were found compared to the positive control. Surviving basal keratinocytes still exhibited the typical elongated morphology (compared to area (A) in figure 5). This indicates that basal keratinocytes in epidermis treated with the astaxanthin-containing product were less affected by UV irradiation.
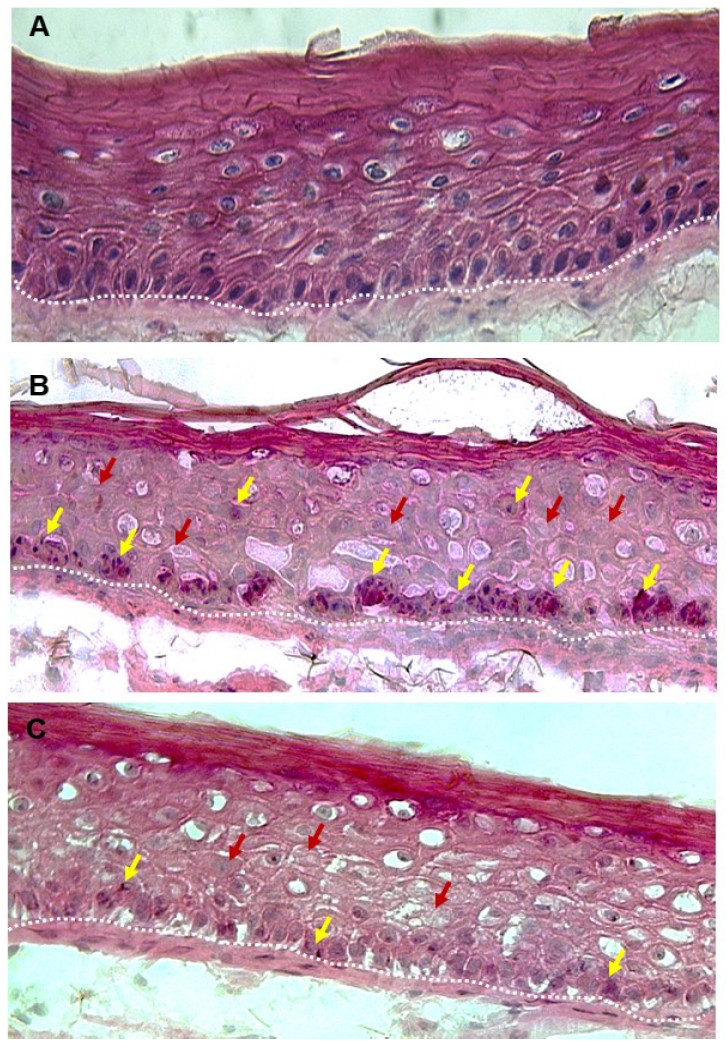
Figure 5: Exemplary representation of UVB-irradiated 3D full skin models with H&E staining after 24 hours. A) untreated control tissue, not irradiated (reference). B) untreated tissue irradiated with 200 mJ/cm² (positive control). C) tissue treated with AstaCos® OL50 after irradiation with 200 mJ/cm². Yellow arrows: sunburned cells (SBCs), red arrows: hydropic cells or tissue, dashed white line: epidermal-dermal transition zone
The results of the described in vitro study therefore showed biological cell protection properties of the astaxanthincontaining test solution against UVB radiation in the irradiated 3D full skin models. These findings supported previous study results showing that astaxanthin can protect against light-induced damage when applied topically (11).
Further clinical studies are needed to determine the longterm effect of topical astaxanthin as an anti-infl ammatory agent against UVB and UVA-induced damage.
Conclusion
Light protection is an important feature of cosmetic approaches against light-induced changes in dermal tissue and accelerated skin ageing. Natural astaxanthin – the red diamond amongst antioxidants – has been shown in vitro and in vivo to be a potent, non-phototoxic antioxidant with strong antinflammatory properties, highly effective in neutralising free radicals. It can protect against light-induced oxidative stress and skin damage without providing a SPF by offering excellent biological cell protection. By suppressing infl ammatory processes caused by harmful UVB radiation, it could prevent further damage to cell structures and later stages of photoageing as well as malignant skin diseases like skin cancer. Therefore, the incorporation of antioxidants like astaxanthin in topical skin care products could offer additional protection against undesirable light-induced skin changes and regenerate the natural oxidative protection mechanism.
References
- S. Davinelli, M. E. Nielsen, and G. Scapagnini, “Astaxanthin in skin health, repair, and disease: A comprehensive review,” Nutrients, vol. 10, no. 4, pp. 1–12, 2018, doi: 10.3390/nu10040522.
- K. Schar?etter-Kochanek et al., “Photoaging of the skin from phenotype to mechanisms,” Exp. Gerontol., vol.35, no. 3, pp. 307–316, 2000, doi: 10.1016/S0531-5565(00)00098-X.
- X. Zhou, Q. Cao, C. Orfi la, J. Zhao, and L. Zhang, “Systematic review and meta-analysis on the effects of astaxanthin on human skin ageing,” Nutrients, vol. 13, no. 9, pp. 1–18, 2021, doi: 10.3390/nu13092917.
- H. W. Lim, I. Kohli, E. Ruvolo, L. Kolbe, and I. H. Hamzavi, “Impact of visible light on skin health: The role of antioxidants and free radical quenchers in skin protection,” J. Am. Acad. Dermatol., vol. 86, no. 3, pp. 27–37, 2022, doi: 10.1016/j.jaad.2021.12.024.
- Y. Nishida, E. Yamashita, and W. Miki, “Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System,” Carotenoid Sci., vol. 11, no. January 2007, pp. 16–20, 2007.
- K. Tominaga, N. Hongo, M. Karato, and E. Yamashita, “Cosmetic benefi ts of astaxanthin on humans subjects,” Acta Biochim. Pol., vol. 59, no. 1, pp. 43–47, 2012, doi: 10.18388/abp.2012_2168.
- N. Ito, S. Seki, and F. Ueda, “The protective role of astaxanthin for UV-induced skin deterioration in healthy people—a randomized, double-blind, placebo-controlled trial,” Nutrients, vol. 10, no. 7, Jul. 2018, doi: 10.3390/nu10070817.
- Dermatest® GmbH, “Bewertung des Erythemschutzes von externen Sonnenschutzmitteln für die menschliche Haut nach EN ISO 24444:2019,” 2022.
- Derma Tronnier GmbH, “In Vitro 3T3 NRU Phototoxicity Test with AstaCos® OL50,” 2021.
- D. Kopera and J. Kim, “UV-protective Properties of Topical of Astaxanthin Half side Controlled Pilot Study,” International Journal of Clinical & Experimental Dermatology, vol. 6, no. 1, pp. 13–16, 2021.
- W. Voss, L. Rüther and G. Schlippe “Physiological/Histological in vitro Expertise, concerning antioxidant capacity, UV-B protection and regeneration in human Phenion® 3D-full thickness skin models and primary human keratinocytes after application of the test product AstaCos® OL50,” DERMATEST GmbH, 2021.

Elisabeth Willeit, MSc.
Elisabeth is Product Development Manager with a focus on Regulatory Affairs at BDI-BioLife Science. In addition to her degree in food product and process development at FH Joanneum, she has professional experience in the food industry. She works as an interface between sales, quality management and product development and deals with regulatory affair issues.